The innermost planet is a lot more interesting than the author realized.
NASA / JHU APL / CARNEGIE INST. OF WASHington
For decades I underestimated you. In the August 2011 issue, I callously lumped you in with the Moon, writing that you’re both “dead” in an unflattering comparison with our solar system’s more active and beautiful orbs.
In lectures, articles, books, and street-corner rants I confidently described you as interesting mostly in comparison with other rocky and senescent orbs. Your story served as a cautionary tale of “don’t let this happen to your planet.” Furious impacts during formation left you with only a metallic core and a flimsy mantle of battered, desiccated rocks from which all of the active and biogenic “good stuff” (water, sulfur, carbon, etc.) was driven off to regroup peacefully elsewhere in the solar system in clouds, ices, oceans, or rocks.
You were a topic of forensic planetary investigation — the time and manner of your death was of interest mainly to planetary-formation theorists. But you were not a place where very much would be happening today, save the knocking about of surface atoms by energetic solar particles.
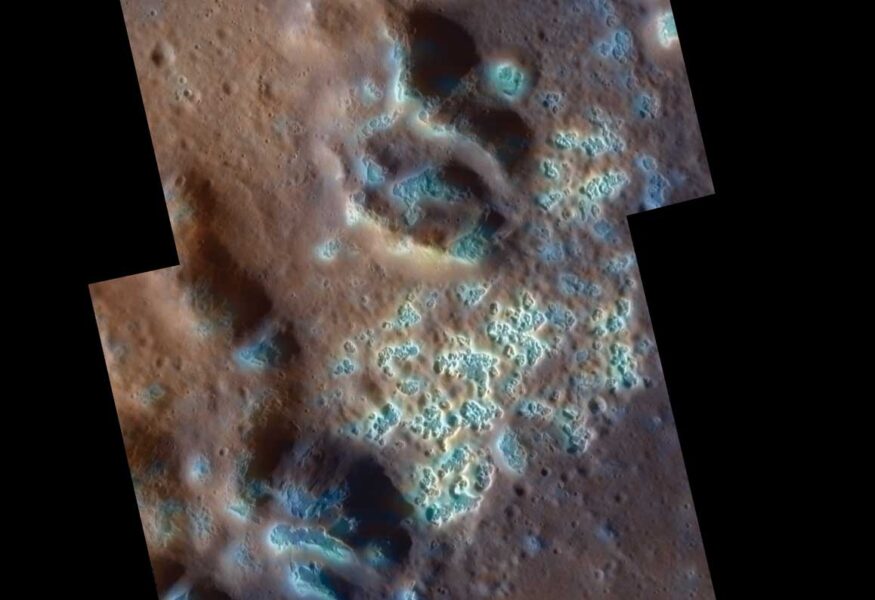
I was at least partially wrong. Mercury is still the outlier at the dense, scorched inner end of our planetary system; vast areas are indeed ancient and cratered. But by giving Mercury its first comprehensive exam, NASA’s sharp-eyed Messenger spacecraft has found things that should not be there. The hot little rock/metal dynamo has been ignoring our theories all along and harboring areas of dynamic change and renewal.
Many of Mercury’s craters are dotted with clusters of small rimless hollows, not old enough to be dented with later craters. What’s happening isn’t exactly clear, but by analogy with similar pits on Mars, it seems that surface material is vaporizing. Perhaps these are widespread deposits of sulfur-rich rocks. If so, what are they doing there? We teach in cosmochemistry class that sulfur is among the most volatile elements, and has no place on such a scorched world. Mercury would have flunked Cosmochemistry 101.
But in science at least, reality rules, so it’s our textbooks that flunk Mercury’s lessons.
I was taught the rules of planetary chemistry by the geniuses who figured them out. My Ph.D. advisor John Lewis derived the theory of “equilibrium condensation,” which gave us our understanding of which type of matter forms at what distance from the Sun: rock and metal in close where it’s hot, ice and gas far from the Sun. Mercury’s surface should therefore lack material that easily vaporizes.
Our minds want to impose order, to find the simple, underlying patterns beneath the surface of the unruly universe. Chemistry pointed toward a well-ordered solar system but physics came along and jumbled everything up. When protoplanets grew almost to planet size, they were tossed around in a mosh pit of mutual gravitation. The clean chemical seating chart was trampled on and some metal and rock were thrown far from the Sun and some ice and water-rich rock was sprinkled throughout the crowd. Perhaps sulfur ended up plentiful on Mercury where we all know it does not belong.
We planetary profs will recover from the indignity, and our newer texts will surely be less wrong. That the Mercurian surface is also mercurial — and surprisingly lovely — reminds us that we have only begun to explore our solar system. Someday our descendants will know if any of our precious ideas endured the relentless bombardment of real data.
This article originally appeared in print in the February 2012 issue of Sky & Telescope. Subscribe to Sky & Telescope.
 0
0
Comments
You must be logged in to post a comment.