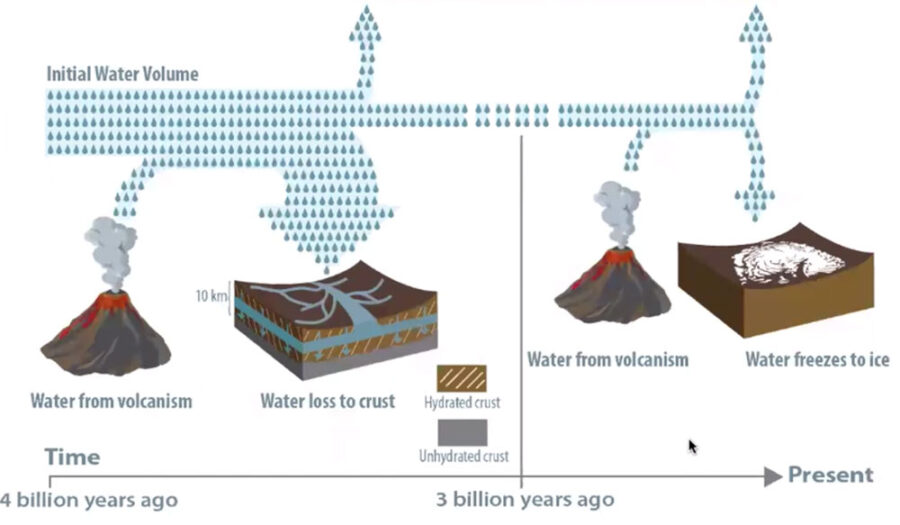
A new study suggests that by 3 billion years ago, most of the Red Planet’s water was locked away in minerals, and it has stayed there ever since.

NASA / Michael Carroll; data source: C. McKay (NASA Ames)
Nearly two dozen orbiters, landers, and rovers sent to Mars have shown that the Red Planet was once quite wet. There’s evidence for enough past water to surround Mars in a global ocean between 100 and 1,500 meters (300 to 5,000 feet) deep.
Now Mars is effectively a desert planet — so where did all that water go?
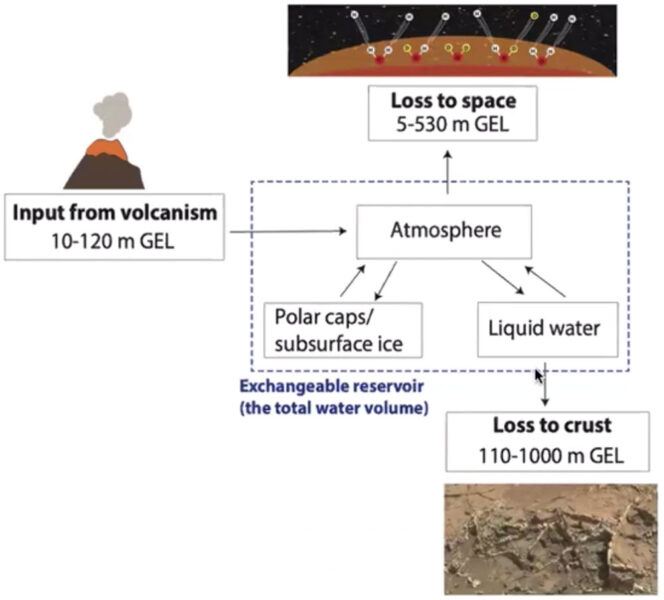
In the March 16th Science, graduate student Eva Scheller and advisor Bethany Ehlmann (both at Caltech) use a model of the Martian water cycle (and water loss) to show that Mars was arid by about 3 billion years ago. But contrary to previous thinking, they argue that most of the water was lost not to space but to the planet itself, trapped in water-loving minerals in the Martian crust.

NASA / JPL-Caltech / MSSS
This happens on Earth, too: Water interacts with surface rocks to form clays, hydrated salts, and other minerals. But Earth has plate tectonics, which continuously drives pieces of its crust into the mantle, releasing water to be outgassed in volcanic eruptions. Geologically dead Mars has no such crustal recycling, so once water reacts with rock or salt to form another mineral, there’s no easy way to reverse the process.
Yet this water-trapping scenario has largely been overlooked in scientists’ modeling of Martian history. A key investigative tool is isotopic ratios, which open a chemical window on the planet’s early chemistry. Water (H2O) usually contains regular ol’ hydrogen but can rarely contain a heavier form called deuterium, which has an extra neutron. Water on Mars has gotten heavier over time; the deuterium-hydrogen ratio in the planet’s thin atmosphere climbed up five to 10 times higher from 4 billion years ago to now.
Previous studies attempting to understand how this ratio has evolved have assumed the water was gradually lost to space over the eons. First, ultraviolet light from the Sun dissociates water molecules, breaking the hydrogens free, then the hydrogen escapes from the top of the atmosphere, albeit gradually. Lighter hydrogen is more likely to escape than deuterium. However, escape hasn’t been able to explain all the water loss. Some scientists have even suggested Mars might harbor a vast but undetected water (or ice) reservoir beneath its surface.
Scheller at al. / Science 2021
So Scheller, Ehlmann, and their colleagues set out to build a model of the Martian water cycle that takes into account volcanic outgassing, exchange with the polar ice caps and other sources of subsurface ice, loss to space, and loss to water-trapping chemistry on the surface. They found that in fact most of the missing water must have been lost to the planet’s crust rather than to space. These surface interactions explain the current deuterium-to-hydrogen ratio without requiring an underground reservoir.
“More than half of Mars’s initial water was sequestered in the crust by 3 billion years ago,” Scheller said at the 52nd Lunar and Planetary Science Conference.
Testing with Perseverance
While the study highlights the importance of surface chemistry in understanding ancient Martian history, just how much water got trapped in crustal rocks is hard to pin down because there’s still a lot we don’t know about the planet’s history.
NASA’s Mars Atmosphere and Volatile EvolutioN (MAVEN) orbiter has measured the current escape rate of hydrogen (and hence water) from the Martian atmosphere, for example, but the rate in the distant past is less certain. If hydrogen has been wafting into space at the same low rate for 3 billion years, then up to 99% of the lost water could be trapped in minerals. But if global dust storms regularly heated the atmosphere, accelerating hydrogen’s escape to space for brief times, then perhaps only 30% of lost water is locked away in the crust.
Scheller et al. / LPSC
“There are so many uncertainties that it’s difficult to come up with a unique scenario,” says Bruce Jakosky (University of Colorado, Boulder), who was not involved in the study but serves as MAVEN’s principal investigator.
Jakosky worked with graduate student Liza Wernicke in assessing the amount of water locked into hydrated minerals on and beneath the surface based on data from two orbiters, Mars Odyssey and Mars Express. Their estimates of locked-away water, published last week in Journal of Geophysical Research: Planets, are consistent with those of Scheller’s team. But Jakosky adds that there are still a lot of open questions in going from the amount of hydrated minerals to understanding the evolution of deuterium abundance.
Geochemist Roger Clark (Planetary Science Institute), who was also not involved in the study, says, “The model is a good start and indicates other factors that previously were not considered.” He thinks amorphous materials, such as the iron oxides common on the planet's red surface, may have played a role in the process, too, something that could be investigated further in lab studies.
The Perseverance rover will be key to testing and building on the model, Ehlmann says, as the rocks in and around Jezero Crater are the oldest ever to be explored by a rover. Perseverance is selecting and packaging up surface samples for eventual return to Earth, and Scheller adds, “measuring those hydrogen isotopes and water content in the lab will allow us to test between the different scenarios presented in the study.”
Life on Mars?
Knowing that Mars has retained much of its water, rather than losing it to space, might be seen as a plus for life on Mars or future human exploration. But since the water has reacted chemically with minerals, some of which may no longer even be near the surface, it’s not readily available for crewed missions. Scheller points out that we’d need to heat the rocks to 300° to 400°C to release the water molecules from their crystalline traps, and we’d need to heat a lot of rock to get a usable amount of water.
While some have suggested nuking Mars (including at Tuesday’s press conference), as Michael Meyer (NASA) put it, “Nuking a planet is usually not a good way to make it more habitable.” And even if water were released on the surface, the same chemical reactions would occur, re-trapping the water in rock within hundreds of years.
 1
1








Comments
Andrew James
March 18, 2021 at 7:53 pm
"Scheller points out that we’d need to heat the rocks to 300° to 400°C to release the water molecules from their crystalline traps, and we’d need to heat a lot of rock to get a usable amount of water."
Iron oxides have complex chemistry. At about 400 °C, Fe2O3 can be partial reduced with hydrogen to produce magnetite via 3 Fe2O3 + H2 → 2 Fe3O4 + H2O).
Mars' abundant alpha-Fe2O3 is red in colour while black magnetic material that contains both Fe(III) and Fe(II).Looking at the Curiosity imaged sediments shows these colours clearly among the clay.
There is also the danger of extracting oxygen and water from iron compounds as they are hazardous substances the fumes or dust can cause pneumoconiosis and other conditions above 10mg/sq.metre. Ferrous Oxide (FeO) in low oxygen atmospheres with long exposure is also damaging and can be combustible as dust. FeO was likely created by volcanism.where below 576C reacts as 4FeO → Fe + Fe3O4 - creating the super-oxygenated iron. the Humans living on Mars will have to face all these hazards.
You must be logged in to post a comment.
You must be logged in to post a comment.